The community of beneficial bacteria that live in our intestines, known as the gut microbiome, are important for the development and function of the immune system. There has been growing evidence that certain probiotics—therapies that introduce beneficial bacteria into the gut—may help alleviate some of the symptoms of intestinal disorders such as Crohn's disease. By studying the interplay between genetic risk factors for Crohn's and the bacteria that populate the gut, researchers at Caltech have discovered a new potential cause for this disorder in some patients—information that may lead to advances in probiotic therapies and personalized medicine.
The results were published online in the May 5 edition of the journal Science.
Previously, scientists had found that patients with Crohn's disease often exhibit alterations in both their genome and their gut microbiome—the diverse collection of bacteria that reside in the intestine. More than 200 genes have been implicated as having a role in the susceptibility to Crohn's. For years, researchers in the field have believed that these are genes that normally function by sensing pathogenic bacteria and deploying an immune response to kill the unwanted microbes; when these genes are defective, the pathogenic bacteria survive, multiply in the gut, and lead to disease.
"While we believe that all of that is true, in this study we were curious to see if some of the genes that are important in sensing pathogenic bacteria may also be important in sensing beneficial bacteria to promote immune health," says the study's first author, Hiutung Chu, a postdoctoral scholar in biology and biological engineering at Caltech. "Typically, the signals from these beneficial commensal microbes promote anti-inflammatory responses that dampen inflammation in the gut. However, mutations in genes that sense and respond to pathogenic bacteria would also impair the response to the beneficial ones. So it's kind of a new spin on the existing dogma."
To figure this out, Chu and her colleagues in the laboratory of Sarkis Mazmanian, the Luis B. and Nelly Soux Professor of Microbiology and a Heritage Principal Investigator, designed several experiments to study how genetic mutations might interrupt the immune-enhancing effects of a known beneficial bacterium, Bacteroides fragilis. The researchers tested their new theory by using B. fragilis to treat mice that had nonfunctional versions of two genes known to play a role in Crohn's disease risk, called ATG16L1 and NOD2.
The researchers found that if just one of these two genes was absent, the mice were unable to develop disease-protective immune cells called regulatory T cells in response to B. fragilis—and that even after treatment with B. fragilis, symptoms in an ATG16L1-deficient mouse model of intestinal disease remained unchanged.
Chu and Mazmanian then obtained blood samples from both healthy patients and patients with Crohn's disease at the Cedars-Sinai Medical Center in Los Angeles. "We could see that certain patients' immune cells responded to Bacteroides fragilis, while immune cells from other patients didn't respond at all," Chu says. "Because the cells from Cedars had already been genotyped, we were able to match up our results with the patients' genotypes: immune cells from individuals with the protective version of ATG16L1 responded to the treatment, but cells from patients who had the mutated version of the gene showed no anti-inflammatory response to B. fragilis."
Mazmanian says the results suggest that the faulty versions of these genes may cause Crohn's disease in two different ways: by being unable to assist in destroying pathogenic bacteria and by preventing the beneficial immune signals usually elicited by "good" bacteria. "What Hiutung has shown is that there are specific bacteria in the human microbiome that appear to utilize the pathways that are encoded by these genes—genes normally involved in killing bacteria—to send beneficial signals to the host," he says.
This work reveals the important relationship between the genome and the microbiome—and it may also one day be used to improve the use of probiotics in clinical trials, Mazmanian says. "For example, our previous work has suggested using B. fragilis as a probiotic treatment for certain disorders. What this new study suggests is that there are certain populations that wouldn't benefit from this treatment because they have this genetic predisposition," he notes. "Right now, clinical trials don't do a good job of identifying which patients might respond best to treatment, but our experiments in mouse models suggest that, conceptually, you could design clinical trials that are more effective."
The research described in the paper, "Gene-Microbiota Interactions Contribute to the Pathogenesis of Inflammatory Bowel Disease," was funded by the National Institutes of Health, the Cedars-Sinai F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, the Lupus Research Institute, the European Union, the Crohn's and Colitis Foundation of America, the Leona M. and Harry B. Helmsley Charitable Trust, and the Heritage Medical Research Institute.
In addition to Chu and Mazmanian, other Caltech coauthors include former graduate students Arya Khosravi (PhD '14) and Yue Shen (PhD '12); research technician assistants Indah Kusumawardhani and Alice Kwon; and Wei-Li Wu, a postdoctoral scholar in biology and biological engineering. Coauthors from other institutions include: Anilton Vasconcelos and Peter Ernst from UC San Diego; Larissa Cunha and Douglas Green from St. Jude Children's Research Hospital in Memphis; Anne Mayer, Amal Kambal, and Herbert Virgin from the Washington University School of Medicine in St. Louis; Stephan Targan and Dermot McGovern from Cedars-Sinai Medical Center; and Ramnik Xavier from Harvard Medical School.
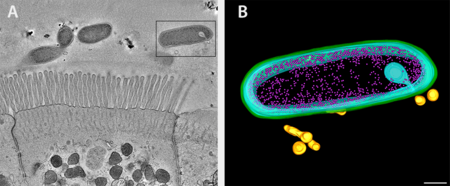 Bacteroides fragilis and intestinal epithelial cells shown as an overview tomogram with the modeled bacterium indicated with a black box (left, fig. A). The model reveals secretion of outer membrane vesicles (right, fig. B), bacterial structures discovered to interact with the immune system via genetic pathways linked to Crohn's disease. Green, outer membrane; light blue, inner membrane; pink, ribosomes; gold; outer membrane vesicles.
Credit: Mark Ladinsky/Greg Donaldson/Caltech
Bacteroides fragilis and intestinal epithelial cells shown as an overview tomogram with the modeled bacterium indicated with a black box (left, fig. A). The model reveals secretion of outer membrane vesicles (right, fig. B), bacterial structures discovered to interact with the immune system via genetic pathways linked to Crohn's disease. Green, outer membrane; light blue, inner membrane; pink, ribosomes; gold; outer membrane vesicles.
Credit: Mark Ladinsky/Greg Donaldson/Caltech

